





 Intelligent Clinical Operations
Intelligent Clinical Operations

 Medical Services
Medical Services

 Regulatory Affairs
Regulatory Affairs

 Pharmacovigilance
Pharmacovigilance

 Data Science
Data Science

 Real-World Insights
Real-World Insights

 Independent Assessment Center
Independent Assessment Center
















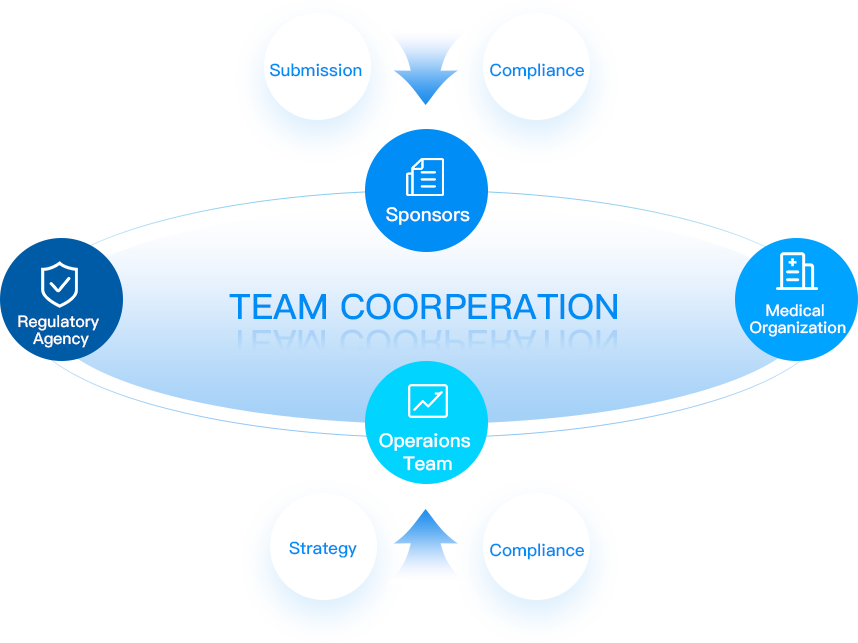
A team with implementation experience to carry out projects in an transparent and compliant manner.
Provide customized services guided by client needs and regulatory requirements.
01CDE communications and consulting
02Human genetic resources applications

01International cooperative scientific research approval
02Collection approval
03Change approval
04Summary report
05Data provided for filing


01
Submission type:
US IND/NDA/ANDA/BLA submissions
EU MAA submissions
02
Service content:
Word file formatted editing and file completion
PDF file editing
eCTD generation and validation
03
Quantity of submitted projects:
30+ NDA;50+ IND;15+ BLA;
25+ ANDA Submissions to FDA;
5+ MAA Submission to EMA;



