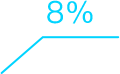
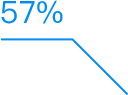
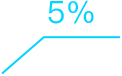
Intelligent Clinical Operations
Intelligent Clinical Operations

 Medical Services
Medical Services

 Regulatory Affairs
Regulatory Affairs

 Pharmacovigilance
Pharmacovigilance

 Data Science
Data Science

 Real-World Insights
Real-World Insights

 Independent Assessment Center
Independent Assessment Center



· Clinical development strategy
· Clinical trial design
· Research strategy and development
· Medical consultation and risk control
· Medical monitoring and medical writing

· IND/NDA/IIT clinical studies and registration support
· KOLs/sites management
· Study patients' eligibility
· Develop and review protocol,ICF,IB,CSR,MMP,CRF,etc.
· High-quality, efficient and timely services

· Protocol design and development for phase IV trials
· IP indications, marketing and strategies
· Brand drugs and medical insurance strategies
· Long-term follow-up and data collection

· Cutting-edge technical support in clinical practice
· Regulatory tracking and implementation
· Personalized strategic marketing plans and implementation