




 Contact Us
Contact Us




· To ensure that the compensation for subjects is "timely paid", effectively protect the rights and interests of subjects, improve patients' experience, enhance patients' satisfaction and reduce drop-out rate, from 2019, TrialNet has gradually adopted new technologies to realize online compensation for subjects.
· Up to now, it has undertaken hundreds of successful cases (the products in some projects have been tested by clinical trials and marketed), mainly registration trials, with Global projects accounting for 20%.







· Electronic prior informed consent
· Electronic primary informed consent
· Face recognition, ID card recognition
· Signing of the informed consent, which is authoritative, accurate and legal as it can be certified online directly via public security channel

· Remote immediate communication
· Continued collection of disease data
· Direct data connection to EDC
· Intelligent patient education with videos
· Reduction of patients' drop-out rate

· Samples are sent for screening after the patient sign the prior informed consent, and the investigator will assess the samples for enrollment
· Map of local examination sites for patients, appointment management (cooperative examination organizations for hemogram and lung functions, etc.)
· Drug distribution map (immediate synchronization of distribution information, 99% prefecture-level city coverage rate)


· Cybersecurity Law
· Data Security Law
· Health Insurance Portability And Accountability Act (HIPAA)
· California Consumer Privacy Act (CCPA)
· General Data Protection Regulation (GDPR)

· Access security
· Data Leak Prevention (DLP)software
· Privacy and security management
· Web Application Firewall (WAF)
· Information security management system
· Security training

· Passing a penetration test
· Situation awareness
· S-SDLC (Secure Software Development Lifecycle)
· Behavioral auditing, etc., for security defense and intrusion detection

· Building an enterprise risk defense network to achieve a strategic risk prevention moat through rules, algorithms, models, strategies and other technologies
· Title 21 CFR Part 11 (International Basic Standard)
· CDISC (Data Interchange Standards)
· GAMP5 (System Verification Standards)



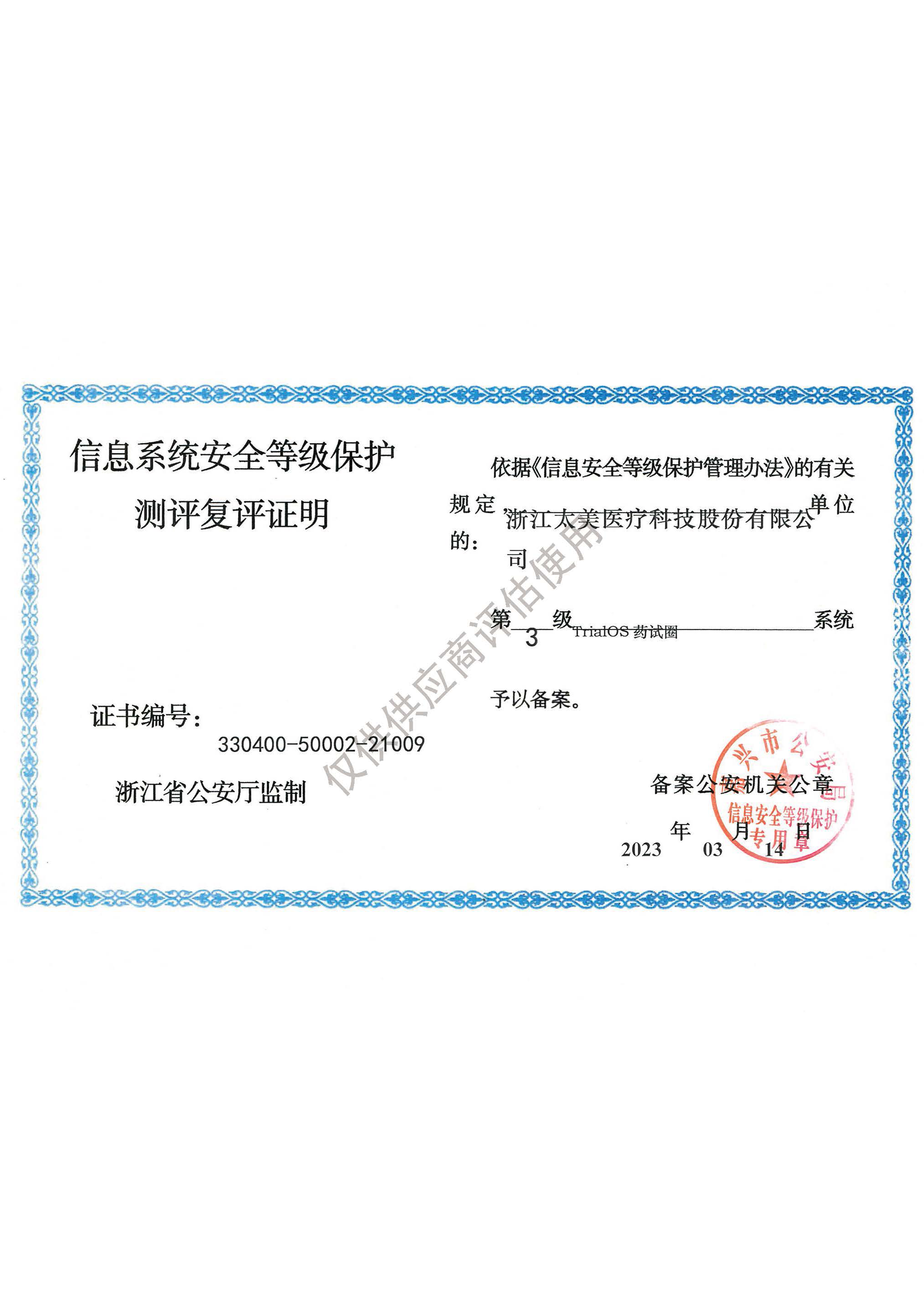
· The Ministry of Public Security of the PRC: Information System Security Level Protection (Level 3)
· China Academy of Information and Communications Technology (CAICT), Ministry of Industry and Information Technology: Credible Cloud Enterprise-level SaaS Service Assessment and Certification
· Internationally certified: SOC2 type1 identification
· First Chinese enterprise among WHO-UMC certified suppliers
WHO Drug coding tool certified by WHO
· The first CDISC system member and registered supplier in China
· National High-tech Enterprise










CDISC: The Clinical Data Interchange Standards Consortium
GAMP: Good Automated Manufacturing Practices

